Due to the production needs, our company needs to regularly check the content of barium ions in the mixed salts of barium chloride and calcium chloride, and timely adjust to the process requirements to ensure smooth production. However, the detection of mixed salts of strontium ions and calcium ions has certain difficulties, and they interfere with each other during the measurement process, which seriously affects the analysis results. Therefore, the process plan should be drawn up, the test analysis should be carried out, and the appropriate analysis method should be finally determined through the test results and accuracy.
1. Test content
On the basis of mass production tests, the following three schemes were developed to analyze cesium chloride after analysis and summary.
(1) In the first scheme volumetric method, a quantitative amount of potassium dichromate and strontium ions are added to an acetic acid-sodium acetate solution to form a yellow strontium chromate precipitate, and an excess potassium dichromate is titrated with ammonium ferrous sulfate. Analytical reagents required: pH5 acetic acid-sodium acetate buffer solution; sulfuric acid solution 2:3; potassium dichromate standard solution 0.1N; ammonium ferrous sulfate standard solution 0.1N; sodium diphenylamine sulfonate indicator 1%.
Weigh 10 g of cerium chloride and calcium chloride mixed salt with known cerium ion content (accurate to 0.0001 g) in a 250 mL volumetric flask, dissolve it, dilute to the mark and shake. Pipette 20mL of the solution in a 250mL volumetric flask, add 10mL acetic acid-sodium acetate buffer solution, 50mL potassium dichromate standard solution, shake for 1-2 minutes, let stand for 5min, dilute with water to the mark and shake. Filter with fast filter paper, measure 100mL of filtrate into 250mL conical flask, add 10mL of 2:3 sulfuric acid solution, 2mL of concentrated phosphoric acid, 1~2 drops of sodium diphenylamine sulfonate indicator, drop to the solution with ammonium ferrous sulfate The magenta turns yellowish green as the end point.
0.40 g of calcium chloride (similar to the calcium chloride content in the mixed salt) was weighed together in the 250 mL volumetric flask along with the above.
(2) The second method gravimetric method, in which potassium dichromate and barium chloride are uniformly formed in the ammonium acetate buffer to form a yellow barium chromate precipitate, and the barium chloride content is calculated according to the mass of the barium chromate precipitate. Chemical reagents required: 50 g/L potassium dichromate; 1:11 hydrochloric acid; 75 g/L ammonium acetate; 1:27 ammonia water. The analytical instruments required for this method are glass mortar, filter plate aperture 5-15 μm and electric drying oven.
Absorb 50mL of the above prepared solution in a 400mL beaker, add 5mL hydrochloric acid solution, 100mL water and 15mL potassium dichromate solution, heat and boil, slowly add 10mL ammonium acetate solution while stirring, keep warm for 5min, continue to be slightly boiling While stirring, 15 mL of ammonia water was slowly added dropwise. It was allowed to stand in a water bath of about 80 ° C for 30 min, rapidly cooled to room temperature, filtered with a glass mortar which had been baked to a constant weight at 130-135 ° C, and washed with distilled water containing a small amount of ammonia water to a chloride-free ion, and the glass sand was removed. The crucible and the precipitate were baked at 130-135 ° C to constant weight.
1.0 g of calcium chloride (similar to the calcium chloride content of the mixed salt) was weighed in a 400 mL beaker and analyzed along with the above.
(3) The third scheme gravimetric method, in hydrochloric acid, strontium ions and sulfate ions react to form barium sulfate precipitate, and the content of barium chloride is calculated according to the mass of barium sulfate precipitate. The required chemical reagents are: hydrochloric acid 1:1; sulfuric acid solution 5:95. The analytical instruments required for this method are porcelain crucibles and muffle furnaces.
Pipe 10mL of the above prepared solution in a 400mL beaker, add 100mL of water, 2mL of hydrochloric acid, heat to near boiling, add 5mL of sulfuric acid solution under constant stirring, keep warm for 5min, and let stand overnight. Filter with ashless filter paper and wash with hot water until there is no chloride ion. The precipitate and filter paper were transferred into a constant weight crucible, placed in a muffle furnace, gradually heated to 850-900 ° C, dried to constant weight, and taken out, and placed in a desiccator to cool to room temperature.
0.20 g of calcium chloride (similar to the calcium chloride content in the mixed salt) was weighed in a 400 mL beaker and analyzed along with the above.
2. Test results and discussion
(1) Test results The results of the above three schemes and the exact content are shown in the attached table.
(2) Analysis and discussion The calculation results of pure calcium chloride simultaneously analyzed by the first scheme are zero calcium chloride, indicating that calcium chloride does not react with potassium dichromate; the second scheme is used for glass mortar of pure calcium chloride analysis. The mass before and after is unchanged, indicating that calcium chloride and potassium dichromate solution have no precipitation in the analysis solution of this condition, that is, calcium chromate is dissolved in weak acid; the third scheme is 0.27% with the analysis result of calcium chloride, which is equivalent to The cesium ion is 0.33%, indicating that calcium ions and sulfate ions have a small amount of precipitation in an acidic solution.
The principles of the first scheme and the second scheme are the same, and both utilize the property that the strontium chromate is hardly soluble and the calcium chromate is dissolved in the weak acid. From the above tests, it can be seen that the two methods of calcium do not affect the determination of strontium. Since strontium chromate is not absolutely insoluble, a slight amount of dissolution during the analysis causes the result to be low. Both methods have their own advantages and disadvantages. The volumetric method is fast and the accuracy is not as good as the weight method. The gravimetric analysis results are accurate, but the analysis process is cumbersome and has a long cycle.
The third scheme calculates the strontium ion content based on the mass of the barium sulfate precipitate, and the result is 2.02% higher, indicating that the scheme is not feasible. The calcium sulphate formed by the simple calcium chloride in the dilute acid is substantially dissolved. However, the precipitation of some calcium sulfate and barium sulfate in the presence of barium ions results in high results.
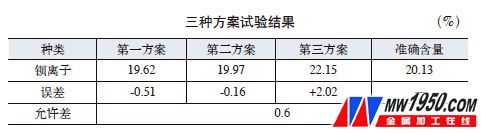
The first and second different analytical methods can meet the production requirements, and the tester can select the appropriate method according to the needs.
3. Conclusion
(1) Analysis of cerium ions in mixed salt by volumetric method, adding potassium dichromate and strontium ions to acetic acid-sodium acetate solution to form yellow strontium chromate precipitate, excess potassium chromite using ammonium ferrous sulfate The standard droplet timing analysis is fast, but the accuracy is not as good as the weight method.
(2) Using the gravimetric method, potassium dichromate and strontium ions uniformly form yellow strontium chromate precipitate in ammonium acetate buffer. The analytical results are accurate according to the mass of strontium chromate precipitation, but the analysis process is cumbersome. The cycle is long.
(3) These two different analytical methods can meet the production requirements, and the tester can choose the appropriate method according to the needs.
About the author: Zhu Yanmei, Zhu Xinfa, Shanghai Tool Factory Co., Ltd. Technology Center; Zhang Qing, Aike (Changzhou) Agricultural Machinery Co., Ltd.
We are manufacturer of Silicone Kitchen Utensils in China, if you want to buy Silicone Cooking Utensils,Silicone Kitchen Utensils Set,Silicone Kitchen Accessories please contact us.All Silicone Parts are heat resistant up to
240 degree celsius or 464 degree fahrenheit. Cooking with the non-stock
silicone utensils set will protect your cookware from any scratch.
Silicone Kitchen Utensils
Silicone Kitchen Utensils,Silicone Cooking Utensils,Silicone Kitchen Utensils Set,Silicone Kitchen Accessories
Yangjiang YJCB Trade Co., Ltd , https://www.cbprokitchen.com
