1. Google Glass, published in the internationally renowned journal “Nature Medicine†published on April 7th, pointed out that the development of long-acting new drugs is the latest strategy to effectively solve the long-term drug treatment failure cases. Aibo Weitai from China has entered the final stage of clinical trials. The phase 3 has been clinically started for 5 months. Xie Dong’s heart has never been relaxed. Since he was in charge of the Nanjing Frontier Biotechnology, he has developed long-term treatment for AIDS. The new drug, Aibo Weitai, announced in February this year that after entering Phase 3 clinical trials, a number of AIDS patients have taken the initiative to come to the hospital to request trials, and none of these patients have developed resistance to traditional anti-AIDS drugs. Medicinal.
The report reviewed three long-acting anti-AIDS drugs currently under clinical trials. Aibo Weitai from China has entered the final stage of clinical trials and is expected to become the world's first long-acting anti-AIDS drug.
With the virus, find the target and put the arrow
35.3 million people living with HIV worldwide, 2.3 million new infections, and 1.6 million people died of AIDS. This is a shocking number that reminds us all the time that AIDS has become a serious threat to human health.
Since the first report of AIDS cases in the United States in 1981, the international community has made tremendous efforts to develop 28 synthetic drugs for the treatment of AIDS, but it is still unable to develop effective drugs for radical and preventive AIDS.
"The reason why AIDS is difficult to prevent and cure is because the HIV virus is very embarrassing. It not only attacks the human immune system, but also is very variable. The anti-AIDS drugs that are now on the market are either due to toxic side effects or due to drug resistance, resulting in many treatments. Failure." Xie Dong, chairman of Nanjing Frontier Biotechnology Co., Ltd. said.
As the first batch of “Thousand Talents†talents introduced in China, Xie Dong is a Ph.D. at Johns Hopkins University. He has worked in many well-known research institutions abroad and has led and participated in the research and development of three new anti-AIDS drugs. In 2002, he returned to domestic entrepreneurship with independent intellectual property technology, and first targeted the AIDS.
"HIV is a highly retrovirus that is prone to genetic variation. The past 30 years of experience have taught us that long-term drug treatment will cause resistance in some patients regardless of the formula, and even some patients will be resistant after a few days of drug abuse. At this time, the effect of re-administration is not satisfactory."
"What should I do with this part of the patient? Is it really impossible to save?" Xie Dong said that there are 12 million infected people in the world receiving drug treatment, and there are about 3 million people with mutations in drug resistance. Up to tens of thousands of people, this is a major hidden danger of global AIDS prevention and treatment.
In recent years, there have been more and more AIDS patients, but the research and development of new anti-AIDS drugs seems to be in a bottleneck period. There are few new drugs in the market, and there are only a handful of new drugs that have been listed and have achieved remarkable results in clinical practice. The global medical market is eager for new types of anti-AIDS drugs to be available as soon as possible.
Xie Dong led the team members to analyze the two proteins gp120 and gp41 on the surface of HIV virus and found that they are the accomplices of HIV invading human T cells. They realized that by controlling these two proteins and preventing them from binding to T cells, they can inhibit the replication and spread of HIV in the human body. They called this new anti-AIDS drug a HIV virus entry inhibitor.
Xie Dong said that from the drug target, Aiboweitai is targeting a new region of the HIV virus gp41 protein, which is effective in inhibiting most HIV viruses, including drug-resistant viruses; Aiboweitai is an original new type of peptide drug that will be metabolized to amino acids in the human body and will not attack human cells. It has shown good safety. From these two points alone, Aibo Weitai has taken a big step ahead of the anti-AIDS drugs that are currently on the market. Even more surprising is that Aiboweitai once a week, compared to the current daily consumption of a drug, can improve patient compliance, significantly reduce the treatment loss caused by not taking the drug on time.
Running against time, loneliness is the biggest opponent
"Only one person, the frontier creatures can't do today." Looking back on Gan Xin, who has been in business for more than ten years, Xie Dong is most grateful to the two "missing eyes" partners - Lu Rongjian, a postdoctoral fellow at Harvard Medical School, engaged in biotechnology. The company's antiviral drug development; Wang Changjin, Ph.D., of the University of Kentucky School of Pharmacy, has been working in new drug research and development, marketing, and business fields for many years.
In order to develop this original innovative medicine for treating AIDS, the three “Thousand Talents†experts have been insisting for more than ten years, and the accumulated investment has reached 100 million yuan.
"People tend to be like this. When you don't have to worry about your life, you start tossing." Wang Changjin said that in the famous companies such as Merck and Pfizer, there are thousands of R&D personnel, and the individual is like a drop of water in the sea. Do the small fish in the big pool, or the big fish in the small pool? In the end, the three chose their own "dig pool".
Every day, new drug research and development is burning money. Along the way, even if the frontier creatures have received the support of various funds, the test of “money shortage†is constantly coming to them.
"I can't remember how many times I didn't pick up the green! First, the executives don't receive the salary. Then, the executives will pay the employees at the end of the family." Wang Changjin said that after ten years, he will go to hundreds of millions of yuan. . However, "we want to make our own achievements and make our own original medicine, we will stick to it."
Before the advent of Aibo Weitai, there were few new intellectual property rights drugs for major diseases in China. The most fundamental reason is that new drug research and development is a difficult system project. It takes a long time, is expensive, is more risky, and must tolerate failure. Because of the high cost, most of the new drug market with high domestic profits and great influence is occupied by multinational companies. Currently, there are 28 anti-AIDS original drugs in the world, but none of them are Chinese.
"Antiviral drug treatment is an effective means to prevent and control the spread of AIDS. There is a constant demand for new drugs in the current situation that cannot be cured and requires lifelong treatment. The state has established an independent new drug research and development system for this major infectious disease, which is not only related to the country. Strategic security and social stability can reduce the huge cost of purchasing new drugs abroad, and also help China to help AIDS-stricken developing countries." Xie Dong said.
From the "Eleventh Five-Year Plan" to the "Twelfth Five-Year Plan" period, the frontier organisms have been continuously supported by the National Major New Drug Creation Technology Major Project; they have entered the "Green Channel" approved by the State Food and Drug Administration, and each time The application for clinical trials has been accelerated; every expert meeting has relevant ministries and commissions to convene experts from various fields... This is an unprecedented rare scene in the development of new drugs in China.
"Zero breakthrough", is expected to pass the life of the baton
In February of this year, in an unknown, narrow and dark conference room in Beijing, the frontier creatures convened the Abbotthai Phase 3 clinical trial kick-off meeting, marking the final “sprint†for the launch of the domestically-made original innovative drug.
The results of the phase 2 clinical trials that have been completed before have shown that the combination of Aiboweitai and Kelizhi is surprisingly good: the drug is highly safe, not only is the HIV virus reduced by 99% in all patients, but The viral load of 56% of patients decreased below the test line. Both data are more than double the standard second-line treatment recommended by the World Health Organization.
Professor Wu Wei, the lead researcher of the trial and director of the Infection Center of You'an Hospital affiliated to Capital Medical University, said: “Aboweitai is one of the few new drug products developed in China with global patents and potential market competitiveness.â€
According to Wu Hao, the new drug has two distinct characteristics: one is long-acting once a week, and the other is effective against a drug-resistant virus. “Abboweitai is the first anti-Ai Ai drug with the goal of effectively treating Chinese patients, marking the improvement of the overall research level of AIDS treatment in China. In the final key research stage of this product, we expect positive patients and clinicians. Participate in the scientific and efficient completion of this important research for the benefit of AIDS patients in China and the world."
In the transition period of building an innovative country, Aibo Weitai entered the phase III clinical trial and took the lead in the development of the global long-acting anti-Ai Xin drug, and is expected to take the lead in achieving a “zero breakthrough†and break the foreign country. It is an important step for pharmaceutical companies to monopolize the anti-Ai New Drug market.
From the initial drug screening to the current clinical trials, Abbott's R&D and production processes are closely aligned with international standards: early efficacy trials were conducted in the United States, and patients in Phase 3 clinical trials were standard first-line treatments. In the case of HIV-infected patients who failed the program, 420 patients were divided into two groups for a controlled trial, and each patient's trial will last for 48 weeks.
It is reported that Abbott's Phase 3 clinical trial will be pushed forward in more than a dozen drug clinical trial institutions nationwide.
"In our eyes, AIDS is not terrible. It is a chronic disease with drug control, provided that our drug development is always ahead of the virus. Fortunately, Abbott's Phase 3 clinical is expected to be in 2014. At the end of the year, the group will be enrolled. In 2015, we will receive 24 weeks of interim data. In 2016, we will declare new drugs, which is expected to become the life relay for Chinese and global AIDS patients,†said Xie Dong.
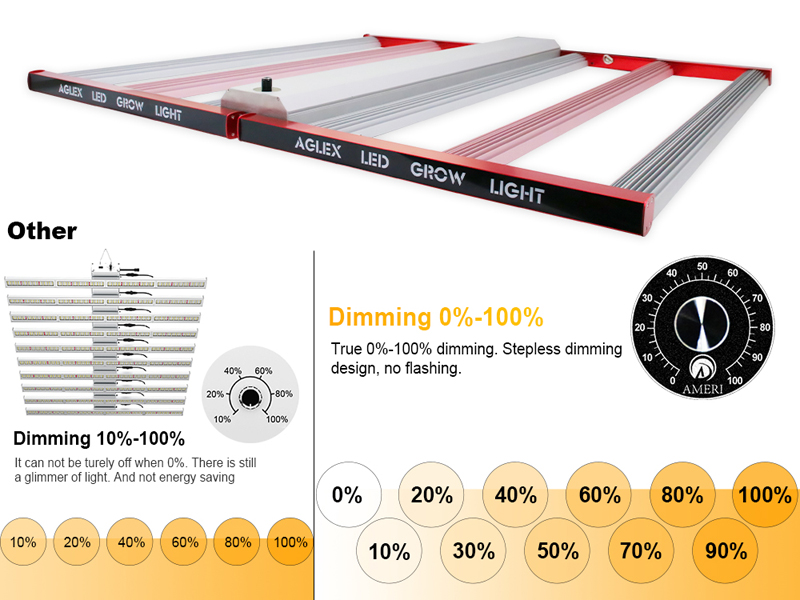
AGLEX has several kinds of dimmable LED grow lights. 0-100% dimming function.
K series (Quantum Board design): K1000 K2000 K4000 LED Grow Light available. K4000 led grow lamp is also foldable.
L series (LED Grow Light Bar design): 700W led Grow Light available. Also it's foldable. Standard grow light 700w is with built-in dimmer, if you need external controller, we can custom for you.
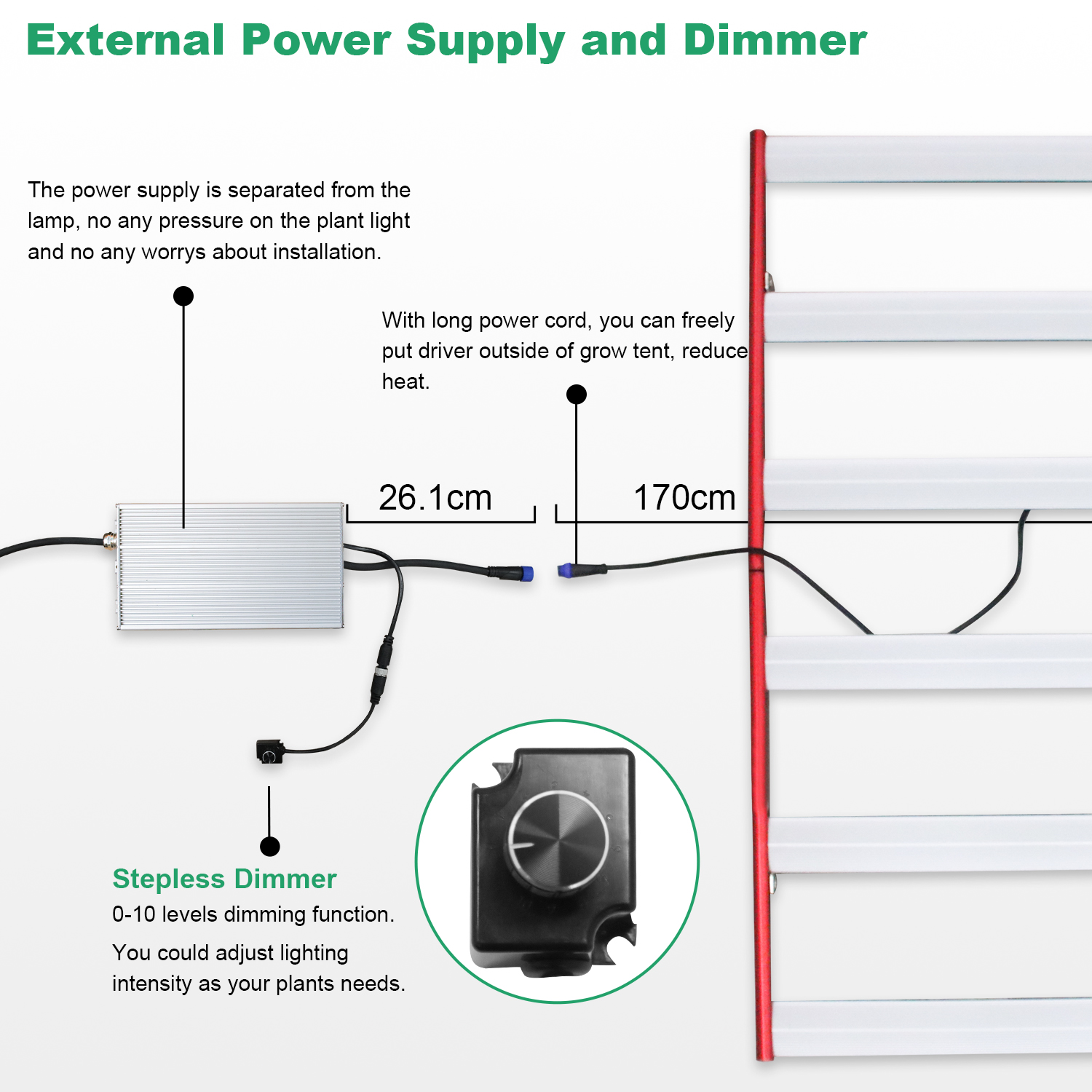
M series (LED Grow Light Bar design): 240W 320W 400W 600W LED Grow Light available. Also it's foldable, but don't like L700 with built-in driver and dimmer, this m series plant grow light is with external driver and dimmer, you could put it outside of tent, turn on/off as your plants needs, no need to step in grow tent. very easy and convenient.
Application:
Suit for marijuana, tomatoes, vegetable, strawberry, pepper, microgreen and herbal plants etc. And suit for all the growing stage.
For grow tent, greenhouse, vertical farming, commercial led grow lighting etc.




Welcome to contact us for quotation.
Grow Light 1000 Watt, Grow Light 1000w, 1000 Watt Grow Light, 1000W Grow Light, qb grow light
Shenzhen Ameri Technology Co., Ltd. , https://www.aglexz.com
